Background: Thrombopoietin receptor agonists (TPO-RAs) (e.g., romiplostim, eltrombopag, avatrombopag) are used to stimulate platelet production in patients with primary immune thrombocytopenia (ITP). While it was previously thought that patients would need to remain on a TPO-RA indefinitely, case reports and cohort studies have shown that some patients have discontinued TPO-RAs while maintaining a hemostatic platelet count. We convened a panel of experts to develop clinical recommendations on when it is appropriate to consider tapering and how to taper TPO-RAs in children and adults with persistent or chronic primary ITP.
Methods: Using a RAND/UCLA modified Delphi panel, we convened 9 hematologists with an average of 25 years of experience and 1 patient representative. Experts were provided with a summary of evidence from 12 case reports, 11 cohort studies, and 2 clinical trial analyses on sustained remission in patients with ITP treated with TPO-RAs. Experts collaboratively developed and then rated (on a 1 to 9 scale) how appropriate it would be to recommend tapering (with the aim of discontinuing) TPO-RA monotherapy in 432 patient scenarios. Each scenario was a simplified patient history which varied by current platelet count, history of bleeding, intensification of treatment, trauma risk, use of anticoagulants or platelet inhibitors, duration of ITP, months on TPO-RA monotherapy, and early platelet response to TPO-RA (Table 1). In addition, the rating form included different ways to taper patients off therapy, how to monitor patients after discontinuation, and how to restart therapy. Ratings were completed independently by each expert before a full-day meeting. Median ratings were grouped into 3 categories (1-3, 4-6, 7-9) and disagreement was defined as ≥2 ratings of 1-3 and ≥2 ratings of 7-9 for a given scenario. During the meeting, discordant ratings were discussed. At the conclusion, experts completed ratings again (final round). Chi-square tests were conducted to determine if each patient characteristic significantly impacted ratings. Final ratings were used to describe the circumstances when it is inappropriate or appropriate to consider tapering TPO-RA monotherapy, how to taper TPO-RAs, how to monitor patients after discontinuation, and how to restart therapy. The panel was double-blinded while work was ongoing: the sponsor did not know the identity of the experts and the experts did not know the identity of the sponsor. The sponsor did not provide input on study design, methods, results, or interpretation of findings.
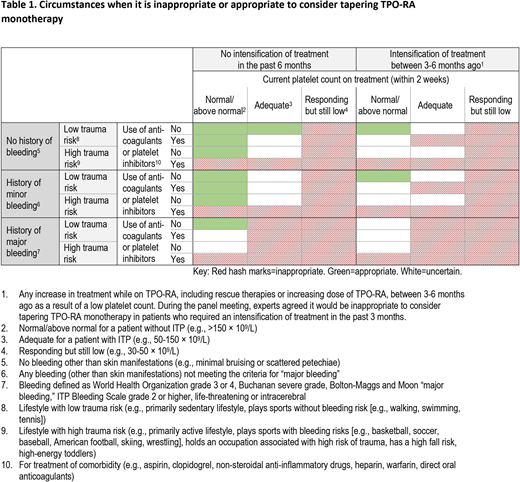
Results: The proportion of items with disagreement decreased from 20% to 10% following the meeting. In the final round, 5 patient characteristics were found to significantly impact ratings and thus the appropriateness of tapering TPO-RA treatment: platelet count (p<0.001), history of bleeding (p=0.001), intensification of treatment (p<0.001), trauma risk (p<0.001), use of anticoagulants or platelet inhibitors (p<0.001). These characteristics were used to describe when it is inappropriate or appropriate to consider tapering TPO-RA monotherapy (Table 1). Experts agreed that it is inappropriate to consider tapering TPO-RA monotherapy in responding patients with low platelet counts, in patients with less than normal but still adequate platelet counts who have a history of major bleeding, or in patients who have a high risk of trauma and are using anticoagulants or platelet inhibitors (regardless of platelet count). It is appropriate to consider tapering TPO-RA monotherapy in patients with normal/above normal platelet counts, no history of major bleeding, and who have not required an intensification of treatment in the past 6 months. Recommendations on how to taper patients off therapy, how to monitor patients after discontinuation, and how to restart therapy were also developed.
Conclusion: A validated methodology was used to assist an expert panel in developing clinical recommendations on when it is inappropriate or appropriate to consider tapering TPO-RA monotherapy and how to safely taper patients off therapy. The guidance reflects areas of greatest agreement based on clinical experience and currently available limited evidence. These recommendations could serve as a guide to clinical care and inform the development and design of clinical trials that prospectively test the safety of tapering TPO-RA monotherapy in patients with ITP.
Cuker:Pfizer: Research Funding; Novartis: Research Funding; Novo Nordisk: Research Funding; Sanofi: Research Funding; Spark: Research Funding; Takeda: Research Funding; Alexion: Research Funding; Bayer: Research Funding; Synergy CRO: Consultancy. Despotovic:Dova: Consultancy; Amgen: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Grace:Novartis: Research Funding; Dova: Membership on an entity's Board of Directors or advisory committees; Agios: Research Funding; Pfizer: Research Funding. Kruse:UCB: Other: Grant and consultancy fee, all paid to PDSA; Rigel: Other: Grant paid to PDSA; Principia: Other: Grant paid to PDSA; Pfizer: Other: Grant and consultancy fee, all paid to PDSA; Argenx: Other: Grant paid to PDSA; Amgen: Other: Grant and honorarium, all paid to PDSA; Novartis: Other: PDSA received payment for recruiting patients to I-WISh and for promoting I-WISh on the globalitp.org website. Grant and consultancy fee, all paid to PDSA outside the submitted work; CSL Behring: Other: Grant paid to PDSA. Lambert:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Platelet Disorder Support Association (PDSA): Consultancy; ClinGen: Honoraria; Principia: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenix: Consultancy; Bayer: Consultancy; ITP Australia: Consultancy; AstraZeneca: Research Funding; Sysmex: Research Funding; Dova: Consultancy, Membership on an entity's Board of Directors or advisory committees; CdLS Foundation: Consultancy; RDMD ITP study: Consultancy; 22qSociety: Consultancy; Octapharma: Consultancy, Research Funding; Educational Concepts in Medicine: Consultancy; Shionogi: Consultancy. Liebman:Janssen: Consultancy; Amgen: Research Funding; Novartis: Honoraria, Research Funding; Kezar: Research Funding; Argenix: Research Funding; Alexion: Other; Genzyme: Consultancy; BMS: Consultancy; Rigel: Consultancy, Research Funding; Portola: Consultancy; Principia Biopharma: Consultancy. Lyons:Novartis: Honoraria; Texas Oncology/US Oncology: Current Employment. McCrae:Dova: Consultancy; Rigel: Consultancy; Momenta Pharmaceuticals: Consultancy; Novartis: Honoraria. Pullarkat:Dova: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genetech: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie, Inc.: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Wasser:Amgen: Consultancy; Biogen: Current equity holder in publicly-traded company, Other: He and his wife have equity ownership; Eli Lilly: Current equity holder in publicly-traded company, Other: He and his wife have equity ownership; Novartis: Honoraria, Speakers Bureau; Pfizer: Current equity holder in publicly-traded company, Other: He and his wife have equity ownership, Research Funding; Incyte: Research Funding; Merck: Current equity holder in publicly-traded company, Other: He and his wife have equity ownership, Research Funding. Beenhouwer:Dr. Beenhouwer reports other from Novartis during the conduct of the study; other from AbbVie, other from Akcea, other from ASPC, other from Amgen, other from AstraZeneca, other from BMS, other from Boston Scientific Corporation, other from Celgene, other: Other: Dr. Beenhouwer reports other from Novartis during the conduct of the study; other from AbbVie, other from Akcea, other from ASPC, other from Amgen, other from AstraZeneca, other from BMS, other from Boston Scientific Corporation, other from Celgene, other. Gibbs:Ms. Gibbs reports other from Novartis during the conduct of the study; other from AbbVie, other from Akcea, other from ASPC, other from Amgen, other from AstraZeneca, other from BMS, other from Boston Scientific Corporation, other from Celgene, other from: Other: SN Gibbs is an employee of the Partnership for Health Analytic Research (PHAR), LLC, which was paid by Novartis to conduct this research.. Yermilov:Dr. Yermilov reports other from Novartis during the conduct of the study; other from AbbVie, other from Akcea, other from ASPC, other from Amgen, other from AstraZeneca, other from BMS, other from Boston Scientific Corporation, other from Celgene, other f: Other: Dr. Yermilov reports other from Novartis during the conduct of the study; other from AbbVie, other from Akcea, other from ASPC, other from Amgen, other from AstraZeneca, other from BMS, other from Boston Scientific Corporation, other from Celgene, other f. Broder:Dr. Broder reports other from Novartis during the conduct of the study; other from AbbVie, other from Akcea, other from ASPC, other from Amgen, other from AstraZeneca, other from BMS, other from Boston Scientific Corporation, other from Celgene, other fro: Other: MS Broder is an employee of the Partnership for Health Analytic Research (PHAR), LLC, which was paid by Novartis to conduct this research..
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal